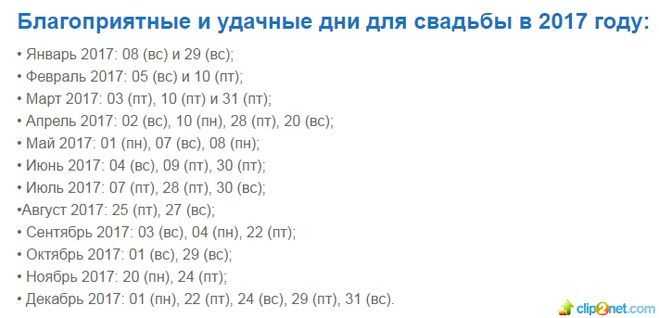
Самостоятельная организация свадьбы – трудоемкий и сложный процесс. С нашими советами он станет проще, и вы не упустите ничего важного!
Что нужно решить в самом начале подготовки?
Если есть возможность, мы рекомендуем координацию свадебного дня отдать профессионалу. Какой бы тщательной ни была предварительная подготовка, хлопот в день свадьбы будет очень и очень много! Координатор избавит вас от решения многих вопросов: от контроля доставки букета невесты до встречи гостей. Однако, если сотрудничество с таким специалистом является проблематичным, то вам придется контролировать все самостоятельно:
- Работу декоратора, флориста и обслуживающего персонала в ресторане
- Встречу и работу ведущего и артистов шоу-программы
- Делать все важные звонки и уточнения по времени прибытия (букет, торт и т.д.)
- Решать форс-мажорные ситуации и все организационные вопросы, которые возникают в течение всего свадебного дня
Самостоятельная подготовка к свадьбе
«Стоит ли обращаться в свадебное агентство или можно организовать свадьбу своими силами?» Этот вопрос невесты задают себе одним из первых, задумываясь о будущем торжестве. Подготовка к свадьбе без организатора отнимает много времени и сил – помните об этом, принимая такое важное решение. Но у него есть и ряд плюсов:
- Полный контроль всех этапов подготовки, личный подбор всех подрядчиков, которые будут задействованы в этом важном празднике – а значит, вы всегда будете в курсе всех нюансов и стадий подготовки.
- Экономия на услугах агентства.
- Привлечение родных и близких к организации. Многие пары хотят всей дружной семьей принимать участие в организации свадьбы – обоюдное решение вопросов, волнительная подготовка и результат, который создали полностью своими руками.
Точно решить, нужен ли вам организатор или вы справитесь своими силами, вам помогут наши советы.